The fuel cell electrode is a thin, catalyst layer where electrochemical reactions take place. The electrodes are usually made of a porous mixture of carbon-supported platinum and ionomer. To catalyze reactions, catalyst particles have contact to both protonic and electronic conductors. There also must be passages for reactants to reach catalyst sites and for reaction products to exit. The contacting point of the reactants, catalyst, and the electrolyte is conventionally referred to as the three-phase interface. To achieve acceptable reaction rates, the effective area of active catalyst sites must be several times higher than the geometric area of the electrode. Therefore, the electrodes are made porous to form a three-dimensional network, in which the three-phase interfaces are located. An illustration of the catalyst, electrolyte, and gas diffusion layer is shown in Figure 1.
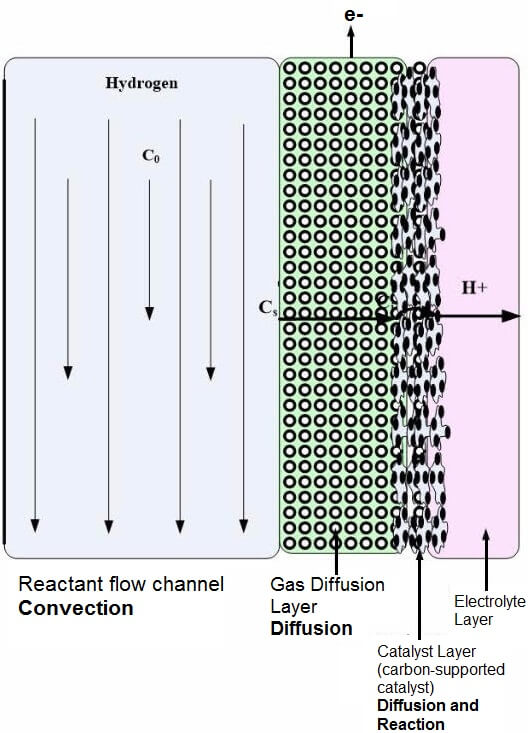
Figure 1: PEM electrolyte, catalyst, and gas diffusion layer.
Most PEM fuel cell developers have chosen the thin-film approach, in which the electrodes are manufactured directly on the membrane surface. Thin-film electrodes offer lower price, higher catalyst surface area, and improved mass transport. The thickness of a thin-film electrode is typically 5 to 15 microns, and the catalyst loading is between 0.1 to 0.5 mg/cm2 for the anode and cathode. Sometimes the anode loading is much lower (0.1 to 0.3 mg/cm2) than the cathode loading.
The catalyst surface area matters more than the weight, so it is important to have small platinum particles (4 nm or smaller) with a large surface area finely dispersed on the surface of the catalyst support, which is typically carbon powders with a high mesoporous area (>75 m2/g).
This layer should be created reasonably thin to minimize cell potential losses due to the rate of proton transport and reactant gas permeation in the depth of the electrocatalyst layer. The metal active surface area should be maximized; therefore, higher Pt/C ratios should be selected (> 40 percent by weight). It has been noted in the literature that the cell’s performance remained unchanged as the Pt/C ratio was varied from 10 to 40 percent with a Pt loading of 0.4 mg/cm2. When the Pt loading was increased beyond 40 percent, the cell performance decreased. Fuel cell performance can be increased by better Platinum utilization in the catalyst layer, instead of increasing the Pt loading.
The type of catalyst needed in a PEMFC or DMFC is dependent upon the type of fuel used. Tolerance to carbon monoxide is an important issue, especially when methanol is supplied to the fuel cell by steam reforming. Methanol reformate can contain as much as 25 percent carbon dioxide (CO2), along with a small amount (1 percent) of carbon monoxide (CO). Fuel cell performance drops with very small amounts of CO concentration (several parts per million), due to the strong chemisorption force of CO onto the catalyst. Two methods of resolving CO poisoning is fuel reforming or catalyst alloying.
If fuel reforming is being used to provide fuel to the fuel cell, the CO concentration must be reduced to at least 100 ppm if the fuel cell type is a PEMFC or PAFC. Some of the methods used for removing CO from the fuel include:
• Selective Oxidation: The reformer fuel is typically mixed with hydrogen and oxygen before the fuel cell is fed into the stack itself. Sometimes this method is used before the fuel is fed to the stack, or within the stack itself. Selective oxidation technologies can reduce CO levels to less than 10 ppm, but this small level of CO is difficult to maintain during actual operating conditions.
• Catalysis: The CO level in the fuel cell can be significantly reduced by passing reforming methanol and oxygen over a Pt aluminum catalyst.
• Hydrogen peroxide bleeding: The use of hydrogen peroxide in an anode humidifier has reduced CO to 100 ppm in a hydrogen feed. This has been accomplished through the decomposition if H2O2 in the humidifier.
One or two other catalysts are typically added to the base catalyst to reduce CO poisoning. Table 1 provides a list of catalyst combinations that have been studied. In the literature, seven catalyst types claim to have equal performance to the typical Pt/C catalyst. These are Pt-Ru/C, Pt-Mo/C, Pt-W/C, Pt-Ru-Mo/C, Pt-Ru-W/C, Pt-Ru-Al4, and Pt-Re-(MgH). Several studies have indicated that only the Pt-Ru catalyst exhibited a cell performance equivalent to the single Pt/C catalyst when exposed to 100 ppm CO. Although Pt/Ru works effectively over the Ru range of 15 to 85 percent, the optimum ratio found was 50:50.
| Single Metal Catalyst | Binary Catalysts | Tertiary Catalysts |
| Pt/C | Pt-Co/C , Pt-Cr/C, Pt-Fe/C, Pt-Ir/C, Pt-Mn/C, Pt-Mo/C, Pt-Ni/C, Pt-Pd/C, Pt-Rh/C, Pt-Ru/C, Pt-V/C, Au-Pd/C | Pt-Ru-Al4 , Pt-Ru-Mo/C, Pt-Ru-Cr/C, Pt-Ru-lr/C, Pt-Ru-Mn/C, Pt-Ru-Co, Pt-Ru-Nb/C, Pt-Ru-Ni/C, Pt-Ru-Pd/C, Pt-Ru-Rh/C, Pt-Ru-W/C, Pt-Ru-Zr/C, Pt-Re-(MgH2) |
Table 1: Anode Catalyst Materials
Some researchers have found that Pt-Mo/C and a non-Pt-based alloy Au-Pd/C can achieve low CO levels. Pt-Mo/C can achieve CO levels as low as < 20 ppm, but for allowable higher levels, the benefit of this catalyst is lessened. Other researchers have found that the traditional Pt/Ru still outperforms these catalysts.
Tertiary catalysts are typically based on a Pt-Ru alloy. A large number of alternative tertiary catalysts have been investigated by scientists. These include Pt-Ru alloys with Ni, Pd, Co, Rh, Ir, Mn, Cr, W, Zr, and Nb. Pt-Ru performed better in the low potential region and Pt-W at high current densities. The tertiary catalysts that performed well with CO gas concentrations of 100 ppm include Pt-Ru-Al4 with no carbon support and PtRe-(MgH2) without carbon support.
The catalyst for the cathode has not been investigated as extensively because it does not have to be CO-tolerant. Cathode catalyst types that have been investigated include Pt- Ni/C, Pt-Co/C, and a nonplatinum-based catalyst produced by pyrolysis of iron acetate, adsorbed on perylenetetracarboxylic dianhydride in Ar:H2:NH3 under ambient conditions.

 Posted by
Posted by





















