Cation exchange membranes (CEMs) are frequently referred to as proton exchange membranes (PEMs) because they are often used in chemical reactions that generate protons. CEMs are used in various applications ranging from proton exchange membrane and microbial fuel cells to chlorine and caustic soda production. The cation exchange membrane (CEM) contains negatively charged functional groups (PO3-, COO–, and C6H4O–) in the membrane backbone, which allows cations to pass through. There are many types of CEM that have been used in the literature, including Nafion©, Fumatech, Aquivion, Xion Composite, polystyrene, glass wool, divinylbenzene, as well as many others. The most common functional groups for CEMs are sulfonic acid, carboxylic acid, and the phosphonic acid groups as shown in Table 1. All three functional group types can exchange hydrogen protons (H+).
Table 1. Common Functional Groups for Cation Exchange Membranes (CEMs).
|
Functional Group |
Formula |
Sodium Salt Form |
|
Sulfonic acid |
R-SO3H |
R-SO3Na |
|
Carboxylic acid |
R-COOH |
R-COONa |
|
Phosphonic acid |
R-PO3H2 |
R-PO3Na2 |
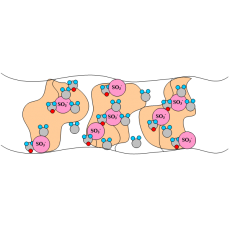
The most common CEM is Nafion©, a copolymer of poly(tetrafluoroethylene) and polysulfonyl fluoride vinyl ether that has high protonic conductivity (0.2 S/cm) and is stable in both oxidative and reductive environments. In this proton-conducting membrane, the sulfonic acid groups are attached to a PTFE-based polymer backbone. The H+ jumps from SO3- site to SO3- site throughout the material, where the H+ emerges on the other side of the membrane. The membrane must remain hydrated to be proton-conductive. This limits the operating temperature to under the boiling point of water and makes water management a key issue when using perflurosulfonic acid membranes. The Nafion© membranes and other PFSA membranes are stable against chemical attack in strong bases, strong oxidizing and reducing acids, H2O2, Cl2, H2, and O2 at temperatures up to 125 ºC. Figure 1 illustrates the SO3- sites in the Nafion© membrane.
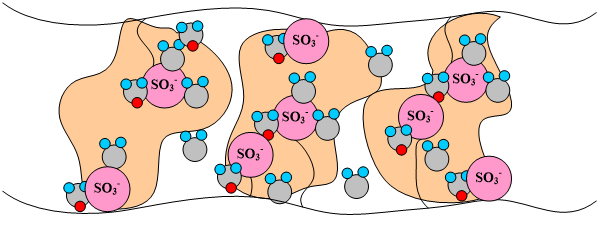
Figure 1. A pictorial illustration of the sulfonic acids groups in Nafion©
The Nafion chemical structure consists of the perfluorosulfonic acid moiety attached to a hydrophobic fluorocarbon backbone and demonstrates a high conductivity not only for hydrogen protons, but for various kinds of cations due to the presence of attached, hydrophilic, negatively charged sulfonate (SO3-) groups. Figure 2 illustrates Nafion’s hydrophobic fluorocarbon backbone.
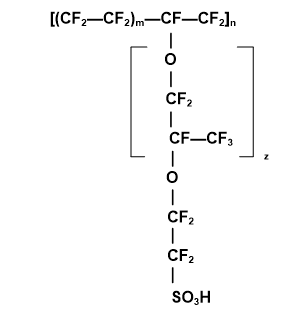
Figure 2. The chemical structure of Nafion©.
Water Uptake in Nafion
The dry membrane absorbs water in order to solvate the acid groups. The initial water content is associated strongly with the sites, and the addition of water causes the water to become less bound to the polymer, which causes the water droplets to aggregate. The water clusters eventually grow and form interconnections with each other. These connections create “water channels”, are transitory and have hydrophobicities comparable to that of the matrix. A transport pathway forms when water clusters are close together and become linked. This percolation phenomenon occurs when the ratio of mol H20/mol SO3- is around 2 (see Figure 3). The next stage occurs when a complete cluster-channel network has formed. In the last stage, the channels are now filled with liquid, and the uptake of the membrane has increased without a change in the chemical potential of water. This phenomenon is known as Schroeder’s Paradox. An illustration of the water uptake of the Nafion© membrane is shown in Figure 3.
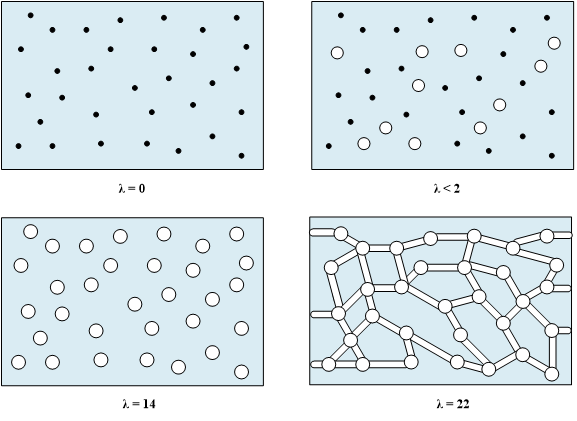
Figure 3. A pictorial illustration of the water uptake of Nafion©.
Schroeder’s paradox is an observed phenomenon that needs to be considered when the membrane is neither fully hydrated nor dehydrated. Depending upon the system, the membrane can be assumed to be fully hydrated or only vapor filled; however, in actuality, the water content is dynamic. Nafion© exhibits a water-uptake isotherm as shown in Figure 4.

Figure 4. Water uptake isotherm at 25˚C showing the effect of Schroder’s Paradox.
Understanding the water content in the membrane is important because ion conductivity is a function of water content. Two transport processes that occur in the polymer membrane are diffusion and the electro-osmotic drag:
1. Electro-osmotic drag happens when hydrogen protons travel through the polymer membrane from the anode to the cathode and carry water molecules with them. The average number of water molecules “dragged” by a single proton is called the electro-osmotic drag coefficient.
2. Back diffusion occurs when the concentration gradient in the cathode drives the diffusion of water through the membrane.
The accumulation of water at the cathode occurs from both the electro-osmotic drag and the production of water at the cathode. The water at the cathode can be either transported to the flow channel through the gas diffusion backing, evaporated through heaters, or diffused through the membrane towards the anode.
Conductivity can be increased through optimal temperature/water balance, thinner electrolytes or membranes, or advanced ion-conductive materials. One of the most effective methods of increasing conductivity is to use a thinner electrolyte layer (or thinner membranes) so that the ions have less distance to travel through the membrane to get to the other side. Thinner membranes are also advantageous because they keep the anode electrode saturated through “back” diffusion of water from the cathode. The amount of water in the membrane must balance the mass transport equations with the protons and water moving through at the necessary speeds.
Limitations
There are also several disadvantages of Nafion and other perfluorosulfonic acid membranes, such as material cost, supporting structure requirements, and temperature-related limitations. For many CEM-based systems, the efficiency increases at higher temperatures, but issues such as membrane dehydration, reduction of ionic conductivity, decreased affinity for water, loss of mechanical strength via softening of the polymer backbone, and increased parasitic losses through high fuel permeation become worse. Nafion is not suitable for neutral pH or solutions containing cationic species such as Na+, K+, and NH4+. These species are more permeable through the membrane than H+; therefore, this process causes a pH increase in the cathode chamber.
Due to Nafion’s limitations, there are numerous other cation exchange membranes that are being investigated. Hydrocarbon alternative membranes may provide some advantages over PFSA membranes, such as cost, commercial availability, and high water uptakes over a wide temperature range, with the absorbed water restricted to the polar groups of polymer chains. Categories of membranes are currently being researched include: (1) perfluorinated, (2) partially fluorinated, (3) non-fluorinated (including hydrocarbon), (4) non-fluorinated (including hydrocarbon) composite, and (5) others. Ultrex is commonly selected as an alternative for CEMs due to its high mechanical stability and affordability. A commonly employed type is Ultrex CMI 7000, a strong acid polymer membrane with a gel polystyrene and a divinylbenzene cross-link structure with numerous sulfonic acid groups. Zirfon and Hyflon are other alternative CEMs. Zirfon consists of 85 wt% of a hydrophilic ZrO2 powder and 15 wt% polysulfone. However, Zirfon has a higher oxygen permeability compared to Nafion, which can be detrimental to certain anodic reactions. Most membranes have degradation temperatures ranging from 250 to 500 ºC, water uptake from 2.5 to 27.5 H2O/SO3H, and conductance from 10 mS/cm to 10 S/cm.
Summary
The cation exchange membrane (CEM) must be a good proton conductor, chemically stable, and able to withstand the temperatures and compression forces of the operating system. Depending upon the system, the ion and water transport and stability properties need to be considered simultaneously. There are many choices for CEM materials, and the decision regarding each choice depends upon many factors including, most importantly, cost and mass manufacturing capabilities.

 Posted by
Posted by








