 Fuel cells often use compressed hydrogen as the fuel; however, many other hydrogen sources can be used with fuel cells. Chemical hydride storage is an alternative method of producing hydrogen via a chemical reaction. These reactions involve chemical hydrides, water, and alcohols. The chemical reactions are not reversible, and the byproducts must be discarded. Hydrogen fuel can also be produced through a chemical reaction with solid “chemical hydrides.” This technique lies somewhere between metal hydrides and reforming. Chemicals such as lithium hydride, lithium aluminum hydride, and sodium borohydride can be combined with water to evolve hydrogen gas exothermically:
Fuel cells often use compressed hydrogen as the fuel; however, many other hydrogen sources can be used with fuel cells. Chemical hydride storage is an alternative method of producing hydrogen via a chemical reaction. These reactions involve chemical hydrides, water, and alcohols. The chemical reactions are not reversible, and the byproducts must be discarded. Hydrogen fuel can also be produced through a chemical reaction with solid “chemical hydrides.” This technique lies somewhere between metal hydrides and reforming. Chemicals such as lithium hydride, lithium aluminum hydride, and sodium borohydride can be combined with water to evolve hydrogen gas exothermically:
LiH + H2O → LiOH + H2 (⌂H = –312 kJ/mol LiH)
LiAlH4 + 4 H2O → LiOH + Al(OH)3 + 4 H2 (⌂H = –727 kJ/mol LiH)
NaBH4 + H2O → NaOH + H3BO3 + 4H2
These compounds are lighter than reversible metal hydrides and release more hydrogen because the hydrogen is liberated from the water reactant. Three example chemical hydrides and their hydrogen storage capabilities are shown in Table 1.
| LiH | LiAlH | NaBH | |
| Hydrogen-to-hydride ratio (wt%) | 25.2 | 21.1 | 21.3 |
| Hydrogen-to-hydride plus stoichiometric water ratio (wt%) | 7.7 | 7.3 | 7.3 |
| Hydrogen-to-system ratio (wt%), assuming 20% additional weight | 6.4 | 6.1 | 6.1 |
| Mass of hydride powder needed for 250 g hydrogen output | 980 g | 1177 g | 1173 g |
| Cost for mass of hydride given above | $268.00 | $503.00 | $178.00 |
Table 1. Chemical Hydride Comparison.
An essential aspect of a chemical hydride system is the weight. The system weight can be estimated using the chemical hydride, water cylinders, mixing device, and control valves. For example, for the LiH to produce 250 grams of hydrogen, 0.98 kg of powered hydride, a 2.2 kg tank of water, and an additional 200 g of control and mixing systems would be needed. Chemical hydrides can be safely contained in small subunits. The hydrides must be stored under nitrogen or argon and protected from water.
Heat generation and cost are the main issues associated with chemical hydrides. The less exothermic of the two reactions (LiAlH4) above produces 182 kJ of heat per mole of H2 released. Depending upon the system design and power, this means that several kilowatts of heat could be generated. This needs to be considered when looking at the overall system design! The cost of metal hydrides is high because they are currently made in small quantities and require greater than 95 percent purity for experimental purposes. The left-over waste solution that results from the chemical reaction can be reprocessed to reduce the costs associated with manufacturing a fresh batch of chemical hydride powder.
Hydrolysis reactions are chemical hydrides that react with water to produce hydrogen. One of the most commonly studied reactions is sodium borohydride and water:
NaBH4 + 2H2O = NaBO2 + 4H2
A common method for activating the reaction is to have an inert stabilizing liquid that protects the hydride from contact with moisture and also makes the hydride pumpable. When fuel is needed, the slurry is mixed with water, and the resulting reaction produces high purity hydrogen. These reactions can be carefully controlled in an aqueous medium using a catalyst and via pH. Although the material hydrogen capacity is high and the release kinetics are fast, this reaction requires water and fuel to be carried onboard separately, and the sodium borohydride to be regenerated off-board. Many aspects of this hydrogen generation method are being investigated, such as cost, life-cycle impact, and regeneration energy requirements. Figure 1 shows an example of a chemical hydride system.
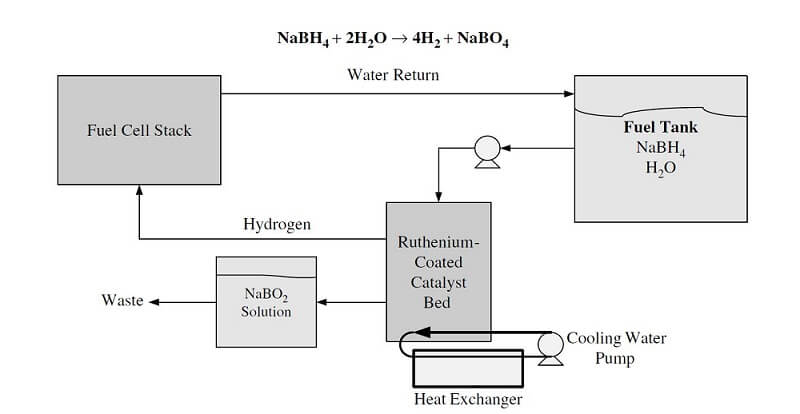
Figure 1. An example chemical hydride system.
Another hydrolysis reaction that is being researched is the reaction of MgH2 with water to form Mg(OH)2 and H2. Particles of MgH2 are contained in a non-aqueous slurry to inhibit the reaction when hydrogen is not required. Material capacities for Mg(OH)2 can be as high as 11 wt.%, but this chemical hydride system also faces similar challenges as the sodium borohydride system.
Hydrogenation and dehydrogenation reactions have been studied for many years, and several systems have excellent potential for hydrogen storage. One of these reactions is the decalin-to-naphthalene reaction, which can release 7.3 wt.% hydrogen at 210 ºC via the reaction:
C10H18 = C10H8 + 5H2
The kinetics of hydrogen evolution is enhanced by a platinum-based or noble metal supported catalyst. Research is currently directed on lowering dehydrogenation temperatures. An advantage of the dehydrogenation systems is that they do not require water. The reactions are endothermic, which means there is no waste heat to be removed while producing hydrogen onboard.
Other chemical approaches that are being investigated include reacting lightweight metal hydrides such as LiH, NaH, and MgH2 with methanol and ethanol (alcoholysis). The hydrogen production from these reactions can be produced at room temperature. Like hydrolysis reactions, alcoholysis reaction products must be recycled off-board the vehicle. Alcohol must be carried onboard the vehicle, which impacts system-level weight, volume, and complexity.
Another new chemical approach is hydrogen generation from ammonia-borane materials via the following reaction:
NH3BH3 = NH2BH2 +H2 = NHBH + H2
This reaction occurs at less than 120 ºC and releases 6.1 wt.% hydrogen. Hydrogen release kinetics and selectivity are improved by incorporating ammonia-borane nanosized particles in a mesoporous scaffold. Table 2 shows a comparison of a few types of chemical hydride storage materials.
| Name | Formula | Percent Hydrogen | Density (kg/L) | Volume (L) to Store 1 kg H2 |
| Lithium hydride | LiH | 12.68 | 0.82 | 0.65 |
| Beryyllium hydride | BeH2 | 18.28 | 0.67 | 8.2 |
| Sodium hydride | NaH | 4.3 | 0.92 | 25.9 |
| Aluminum hydride | AlH3 | 10.8 | 1.3 | 7.1 |
| Potassium hydride | KH | 2.51 | 1.47 | 27.1 |
| Calcium hydride | CaH2 | 5.0 | 1.9 | 1.1 |
| Lithium borohydride | LiBH4 | 18.51 | 0.67 | 8.0 |
| Sodium borohydride | NaBH4 | 10.58 | 1.0 | 9.5 |
| Aluminum borohydride | Al(BH4)3 | 16.91 | 0.545 | 11 |
| Palladium hydride | Pd2H | 0.471 | 10.78 | 20 |
Table 2. Chemical Hydride Storage Materials.
There are many fuel options for different fuel cell types and systems. The cleanest fuel type is pure hydrogen, and there are multiple methods for generating pure hydrogen in-situ. Alternative methods of producing hydrogen are chemical hydrides, hydrolysis reactions, hydrogeneration/dehydrogenation reactions, and alcoholysis reactions. These methods of generating hydrogen may prove advantageous for creating hydrogen for fuel cells and other future alternative energy technologies.

 Posted by
Posted by

















Enter the code in the box below: