 Fuel cells are not limited to pure hydrogen gas as fuel. Each type of fuel cell stack has different fuel tolerances. The lower the operating temperature of the stack, the stricter the requirements for pure fuel. For fuels other than pure hydrogen, an external fuel processing system may be required. The complexity of this system is dependent on the type of fuel cell and fuel used. The fuel processing system usually consists of a series of catalytic chemical reactors that convert the fuel into hydrogen. Fuel fed to a PAFC should be hydrogen-rich and contain less than 0.5 percent carbon monoxide. Fuels used for a PEMFC are preferably carbon monoxide free. Other fuel cell types such as MCFCs and SOFCs operate at temperatures high enough to allow internal reforming. Since PEMFC and PAFCs have stricter fuel processing requirements, these require a fuel processing subsystem. Most of these subsystems consist of three processes: fuel reforming, water shift reaction, and carbon monoxide cleanup.
Fuel cells are not limited to pure hydrogen gas as fuel. Each type of fuel cell stack has different fuel tolerances. The lower the operating temperature of the stack, the stricter the requirements for pure fuel. For fuels other than pure hydrogen, an external fuel processing system may be required. The complexity of this system is dependent on the type of fuel cell and fuel used. The fuel processing system usually consists of a series of catalytic chemical reactors that convert the fuel into hydrogen. Fuel fed to a PAFC should be hydrogen-rich and contain less than 0.5 percent carbon monoxide. Fuels used for a PEMFC are preferably carbon monoxide free. Other fuel cell types such as MCFCs and SOFCs operate at temperatures high enough to allow internal reforming. Since PEMFC and PAFCs have stricter fuel processing requirements, these require a fuel processing subsystem. Most of these subsystems consist of three processes: fuel reforming, water shift reaction, and carbon monoxide cleanup.
Liquid hydrocarbon fuels contain more energy per unit volume than hydrogen and are cheaply available. Methanol produces less carbon dioxide than gasoline and reduces long-term oil dependence. A comparison of gravimetric and volumetric energy densities of hydrocarbon-based fuels are shown in Table 1.
| Fuel | By Mass | By Volume |
| Hydrogen Gas, Atmospheric Pressure | 120 MJ/kg | 11 kJ/L @ STP |
| Compressed Hydrogen Gas, 3600 psi | 120 MJ/kg | 2700 kJ/L @ 3600 psi |
| Gasoline | 44 MJ/kg | 31800 kJ/L in liquid |
| Methanol | 20 MJ/kg | 15900 kJ/L liquid |
| Natural Gas (pure methane) | 50 MJ/kg | 36 kJ/L @ STP |
| Compressed Methane, 3600 psi | 50 MJ/kg | 8700 kJ/L @ 3600 psi |
Table 1. Fuel Gravimetric and Volumetric Energy Densities, Lower Heating Value Basis.
If you want to use an impure fuel stream, the fuel cell must be designed for alternative fuel types. Fuel cells can be designed to accept an anode fuel stream with as little as 40 percent hydrogen for partial oxidation or 75 percent hydrogen for steam reforming. Lower percentages of hydrogen reduce the fuel cell efficiency. If water is required for the reaction, it must be either be carried to supply the water-gas shift reaction (and any steam reformer) or recirculated from the exhaust. The total water needed is on the order of 3 grams per gram of H2 for partial oxidation, and 4.5 grams per gram of H2 for steam reforming.
CO poisoning is an important issue for polymer electrolyte membrane fuel cells. CO poisons the platinum on the electrode, which reduces the voltage at a given current density. For the same power output, a fuel cell running off reformed hydrogen must be sized larger. It is difficult to eliminate CO from the reformer exhaust, and fuel cells can only tolerate, at most, 50 ppm CO before efficiency drops dramatically. Therefore, a final clean-up step is required even after the water-gas shift. A preferential oxidizer (PROX) is needed to perform CO removal.
The amount of hydrogen that can be produced for reforming various hydrocarbons is listed in Table 2 for both steam reforming and partial oxidation. Both processes include a water gas shift reaction to create more hydrogen from shifting carbon monoxide.
| Steam Reforming | Partial Oxidation | ||||||
| Fuel | Formula | Wt% H2 | g H2 per Lfuel | Moles H2O per mol fuel | Wt% H2 | g H2 per Lfuel | Moles H2O per mol fuel |
| Methanol | CH3OH | 19% | 150 | 1.0 | 13% | 100 | 0.0 |
| Ethanol | C2H5OH | 26% | 209 | 3.0 | 22% | 168 | 2.0 |
| Methane (LNG) | CH4 | 50% | 205 | 2.0 | 38% | 151 | 1.0 |
| Gasoline | C8H15.4 | 43% | 301 | 16.3 | 28% | 200 | 8.1 |
| Diesel Fuel | C14H25.5 | 42% | 357 | 28.3 | 28% | 231 | 14.2 |
Table 2. Hydrogen Produced from Hydrocarbon Reformation.
Excellent overall weight fractions and hydrogen densities can be achieved in the fuel, but this does not include the additional weight of reformer equipment required or the extra water needed. To compare reforming process effectiveness, the hydrogen percent and the steam-to-carbon ratio (S/C) can be used. The H2 percent is the molar percentage of H2 in the reformate stream at the outlet of the fuel reformer:
where nH2 is the number of moles produced by the fuel reformer, and ntot is the total number of moles of all gases at the outlet. The steam-to-carbon ratio is the number of moles of molecular water (nH2O) to the moles of atomic carbon (nc) in a fuel:
Most fossil fuel–based liquids have sulfur compounds that need to be removed before any further fuel processing can be performed. The fuel cell catalysts and reforming catalysts can occur with sulfur levels as low as 0.2 ppm. For adequate fuel processor lifetime, the desulphurization step is critical. Levels as low as 1 ppb are enough to poison a PEM fuel cell anode catalyst permanently. Gasoline typically has about 300 to 400 ppm of sulfur compounds. Over the past few years, limits have been put on the amount of sulfur to help reduce emissions from the vehicles. The design of a desulphurization system for a fuel cell stack must be carefully considered. It is common practice to use a desulphurization (HDS) reactor where organic sulfur compounds are converted into hydrosulfide through nickel-molybdenum oxide or cobalt-molybdenum oxide catalysts through a reaction of the type:
(C2H5)2S + 2H2 → 2C2H6 + H2S
The rate of hydrogenolysis increases with increasing temperatures between 300 to 400 ºC. Sulfur compounds such as thiophene (C4H4S) and tetrahydrothiophene (THT) (C4H8O2S) have a slower reaction rate and are absorbed onto a bed of zinc oxide forming zinc sulfide:
H2S +ZnO → ZnS +H2O
HDS as a means of removing sulfur is suited to PEM or PAFC systems. This system cannot be applied easily to SOFC or MCFC systems because there is not enough excess hydrogen for HDS.
Steam reforming is an endothermic process that combines the fuel with steam to produce products. The steam-reforming equivalent of the reaction for methane and a generic hydrocarbon are as follows:
CH4 + H2O → CO + 3H2 (⌂H = +206.2 kJ/mol)
CnHm +nH2O → nCO + (m/2 + n) H2
The next step in either process is the water-gas shift reaction. Most of the remaining carbon monoxide reacts with water to produce additional hydrogen. A typical conversion is from 7.1 percent CO in a steam reformer’s output (or 46.1 percent from a partial oxidation reactor) to 0.5 percent coming out of the water-gas shift reactor.
CO + H2O → CO2 + H2 (⌂H = –41.2 kJ/mol)
These reactions are usually carried out using a nickel support catalyst at temperatures over 500 ºC. The product gas is a mixture of carbon monoxide, carbon dioxide, hydrogen, methane, and steam. The product composition is determined by the temperature of the reactor, the operating pressure, the composition of the feed gas, and the amount of steam fed into the reactor. A steam reforming system is more efficient because waste heat from the later processes can be recycled as input into the endothermic steam reforming process. Steam reformers also produce more hydrogen because it also comes from the water. For fuel cell systems that require low amounts of CO, further processing of the fuel will be required. When natural gas is reformed, the reaction eventually becomes exothermic as the temperature is lowered. The reverse of the reaction becomes favored, and the formation of methane begins to dominate. Figure 1 shows an example of a methanol steam reforming/PEMFC system.
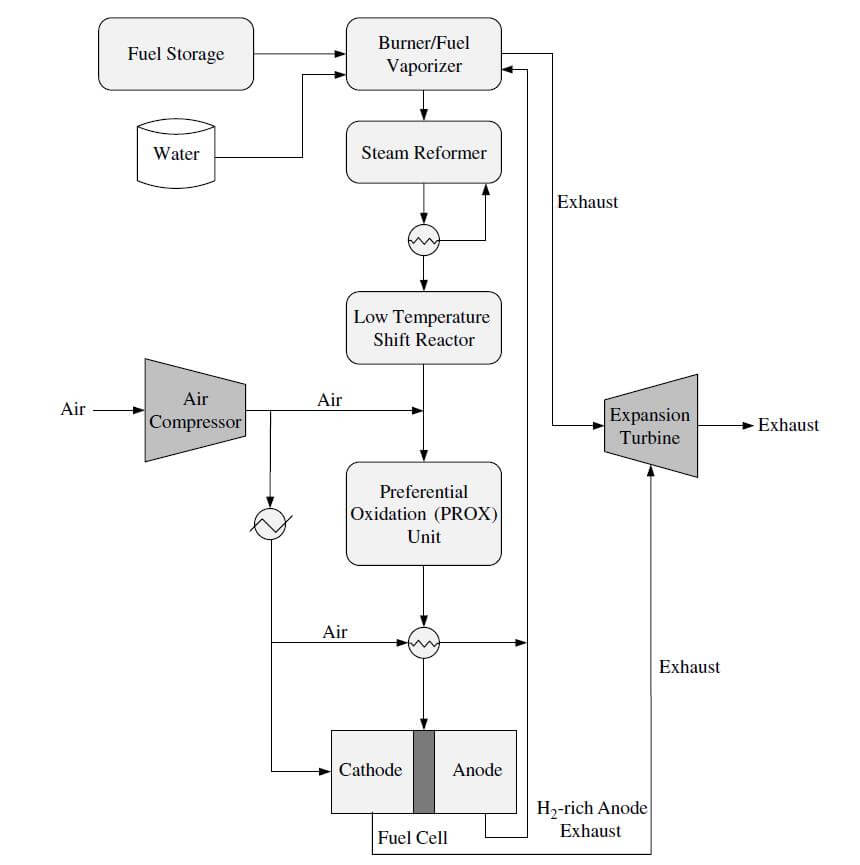
Figure 1. A methanol steam reforming/PEMFC system.
Carbon formation is a potential risk in fuel gas from reforming systems. In the absence of air or steam, natural gas will decompose when heated above 650 ºC, yielding reactions of the type:
CH4 → C + 2H2 (⌂H = +75.0 kJ/mol)
Higher molecular weight hydrocarbons tend to decompose more rapidly than methane; therefore, the risk of carbon formation is greater. The Boudouard reaction occurs from a disproportionate amount of carbon monoxide:
2CO → C + CO2
The risk of extra carbon from the last two reactions can be reduced by adding steam to the fuel stream. Steam can also lead to the following reaction:
C + H2O → CO + H2
Carbon formation on steam reforming catalysts has been the subject of great study. The carbon that poisons the catalyst attaches to the nickel crystallites in the catalyst. It can take only seconds to poison the catalyst and cause the reactor to be plugged.
Many internal reforming concepts have been created and applied for use with molten carbonate or solid oxide fuel cell stacks. The heat required to reform low molecular weight hydrocarbons can be provided via the heat generated by the stack. There are two main approaches to internal reforming in fuel cells: direct and indirect reforming. The advantages of internal reforming compared with external reforming are (1) reduced system cost because an external reformer is not needed, (2) less steam required, (3) more even hydrogen distribution in the cell, (4) high conversion of methane, and (5) higher system efficiency.
Indirect internal reforming involves the conversion of methane by reformers in close contact with the stack. For example, in specific designs, there are plate reformers next to each cell. A variation of this type of reforming is placing the reforming catalyst in the gas distribution path of each cell. An illustration of indirect internal reforming is shown in Figure 2.
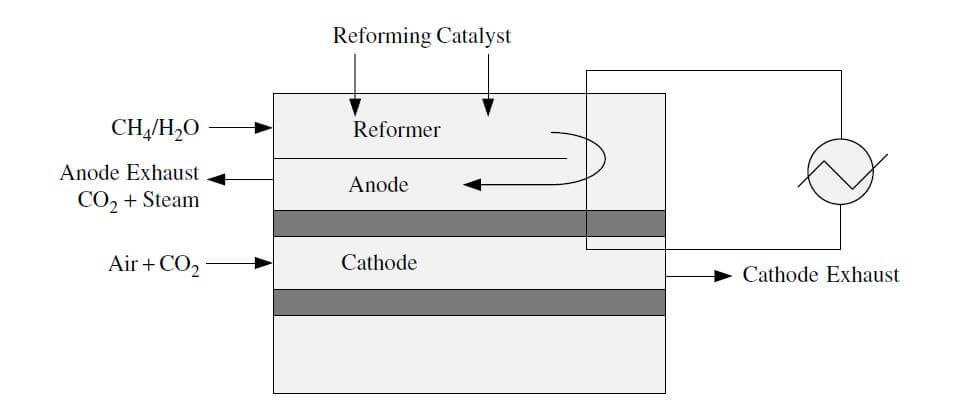
Figure 2. Indirect internal reforming.
In direct internal reforming, the fuel reforming takes place in the anode compartment of the fuel cell. This can be accomplished by placing the catalyst directly in the fuel channels, or the reaction takes place directly in the anode. This method utilizes the heat from the anode and the steam from the electrochemical reaction in the MCFC or the SOFC. Gases that have been used in direct reforming stacks include natural gas, naphtha, kerosene, and coal gases. Figure 3 shows a diagram of direct internal reforming.
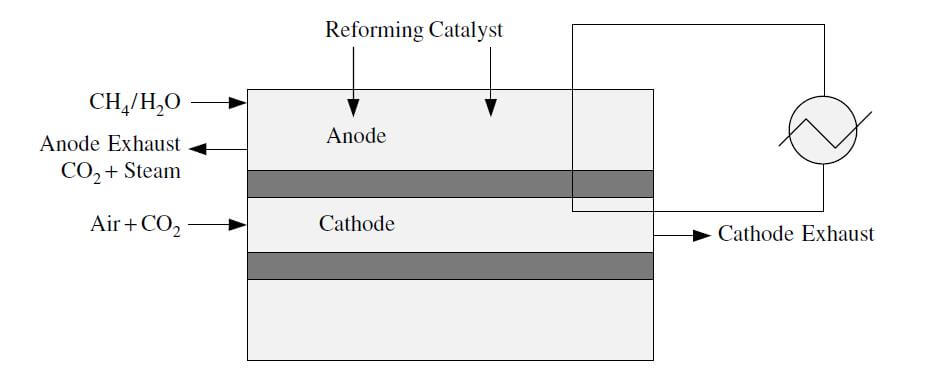
Figure 3. Direct internal reforming.
Direct hydrocarbon oxidation converts methane to carbon dioxide and water with high efficiency. The major issue with direct hydrocarbon oxidation is carbon formation. This method has been investigated on novel ceramic anodes for certain types of SOFCs. Development of this process is still in the research and development stages.
Partial oxidation is a simpler system because of less heat integration and water management issues. It has lower capital costs for this reason. Partial oxidation reformers also have superior startup times, fuel flexibility, and may have faster response times. The next step in either process is the water-gas shift reaction. Most of the remaining carbon monoxide reacts with the water to produce additional hydrogen. A typical conversion is from 7.1 percent CO in a steam reformer’s output (or 46.1 percent in from a partial oxidation reactor) to 0.5 percent coming out of the water-gas shift reactor.
CO + H2O → CO2 + H2 (⌂H = –41.2 kJ/mol)
An example of the POX/PEMFC system is shown in Figure 4.
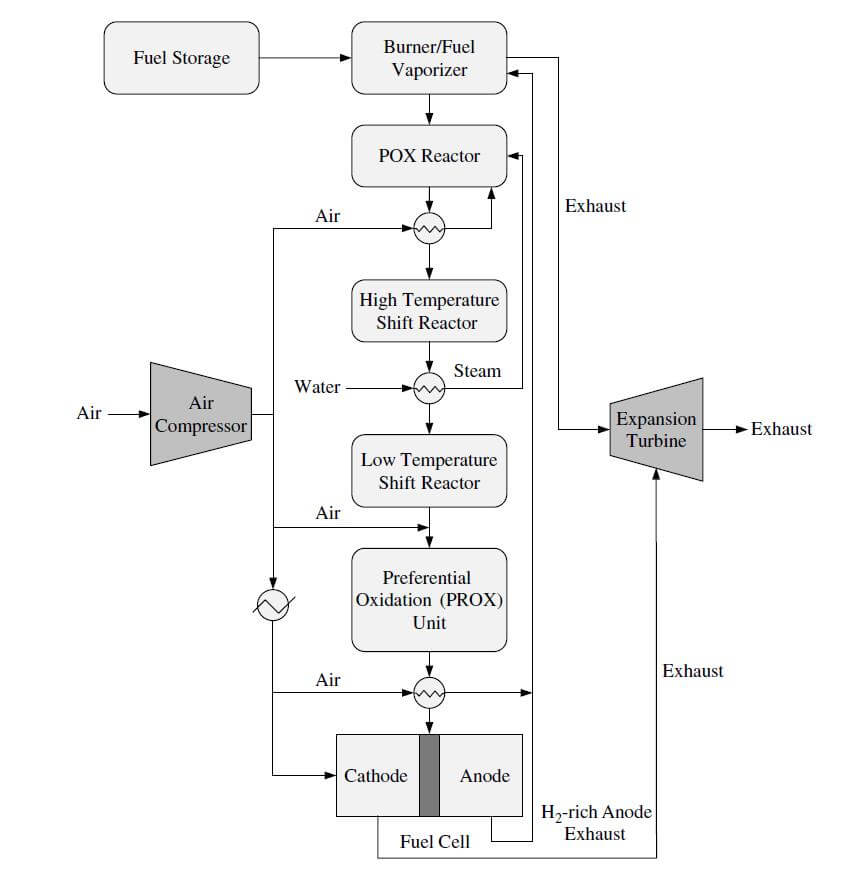
Figure 4. The POX/PEMFC system.
Pyrolysis is the process of heating hydrocarbons in the absence of air. The hydrocarbon is cracked into hydrogen and solid carbon. The process usually uses light hydrocarbons, and the hydrogen produced is often very pure. One of the challenges in pyrolysis is the removal of carbon from the reactor. A commonly used method is turning off the reactor and allowing air into it to form carbon dioxide. Precise control of this process is critical to producing large amounts of hydrogen, and for preventing too much carbon buildup—which can poison the catalyst.
Methanol reforming is the leading reforming technology candidate for PEM fuel cells because methanol is a liquid with high efficiency and energy density, and because it is easier to reform than gasoline. It contains 12.5 percent hydrogen by weight. The overall efficiencies of these systems are 89 percent. Fuel cells running on reformate cannot be dead-ended, so hydrogen utilization at the anode decreases to about 85 percent, for a total efficiency of 76 percent. The reduced hydrogen content in the reformate output (when compared to pure hydrogen) reduces the voltage of the fuel cell by approximately 0.128 volts per A/cm2 or roughly 20 percent at maximum power output.
| Reformer volume | 7.2 L |
| Reformer weight | 9.5 kg + cleanup unit weight |
| Hydrogen output after cleanup | 1.36 L/s |
| Hydrogen output | 0.061 mol/s |
| Fraction of hydrogen in output | 43% |
| Fraction of CO in output | 8 +/– 5 ppm |
| Overall efficiency | 76% |
| Output per mole of methanol | 2.3 mol H2 |
Table 3. Example Reformer Performance.
At this production rate, 55 moles of methanol would be needed for the required 250 grams of hydrogen gas output. This equals 1.8 kg of methanol and a volume of 2.2 L—much less than a compressed gas cylinder or metal hydride device. The total volume is only 9.4 L, and the total weight of the system is 11.3 kg plus the weight of the PROX.
There are many fuel alternatives for fuel cells. The cleanest fuel type is hydrogen, but many fuel cell designs utilize other fuels because of the availability or perceived safety of other fuels. Common fuels include hydrogen, methanol, ethanol, ammonia, natural gas, and gasoline. Many of these fuels can be directly fed to the fuel cell, but other fuels require pre-processing before being introduced into the stack. Many different systems can be used for the pre-processing steps. Some standard methods of processing hydrogen include steam reforming, internal reforming, partial oxidation, and methanol reforming. When reformers are used, the engineer must consider the heat generated, and the effect on the fuel cell stack. The use of fuels other than hydrogen may be beneficial in the near term, but the goal of fuel cell technology is to use pure hydrogen from renewable sources of energy other than fossil fuels.

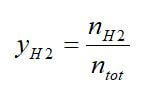
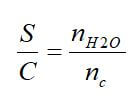
 Posted by
Posted by
















Enter the code in the box below: