There are many novel hydrogen methods that are currently being investigated that offer the potential for higher energy density than conventional methods. These include hydrogen storage in carbon nanotubes. Carbon nanotubes are unique structures with exceptional electronic and mechanical properties. A carbon nanotube is a hexagonal network of carbon atoms that have often been rolled into a cylinder. The size of these structures is often a nanometer across and tens of microns long. The definition of a carbon nanotube is:
| Large molecules of pure carbon that are long and thin tubes. They are 100 times stronger than steel at 1/6 of the weight. They can be used as electrical and heat conductors, hydrogen storage, as well as many other applications. |
Carbon nanotubes are potentially useful for many applications in nanotechnology, electronics, optics, and materials science for their electrical conductivity, heat conductivity and high strength. Under an electron microscope, the nanotube material looks like a mat of carbon ropes as shown in Figure 1. These ropes are 10 to 20 nm across and up to 100 microns long. Each rope consists of a bundle of single-wall nanotubes aligned in a single direction.
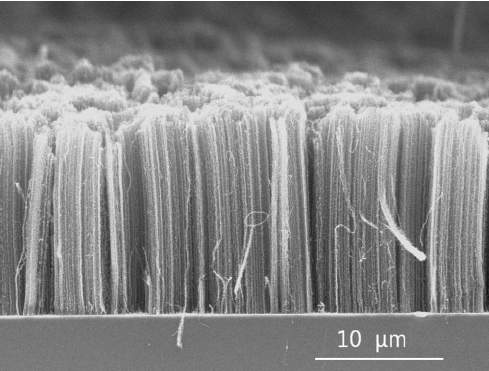
Figure 1. SEM image of aligned carbon nanotubes approximately 20 um long1
There are many types of carbon nanotubes, but the ones commonly used can be categorized as graphitic nanofibers, single-walled nanotubes (SWNTs) or multi-walled nanotubes (MWNTs). Graphitic nanofibers are made by breaking hydrocarbons or carbon monoxide over suitable cataltsys, and the structures can be a platelet, ribbon or herringbone arrangement. Graphitic nanofibers range from 50 nm to 100 nm in length and 5 to 100 nm in diameter.
Single-walled nanotubes (SWNTs) are important because they exhibit exceptional electrical properties and are a good candidate for miniaturizing electronics. The SWNTs can replace electric wires on the micro-electromechanical (MEMs) scale. The main hindrance to the widespread use is that they are expensive to produce.
Multi-walled nanotubes (MWNT) consist of multiple layers of carbon nanotubes to form a cylindrical shape. These structures look like rolls of cylinders or like newspaper pages rolled up in into a cylinder. Both single-walled and multiwalled carbon nanotubes have highly ordered, smooth graphitic surfaces. These are usually prepared using an electric arc drawn between two carbon electrodes, laser ablation, or chemical vapor deposition. Figure 2 shows a schematic of a single-walled and a multiwalled carbon nanotube.
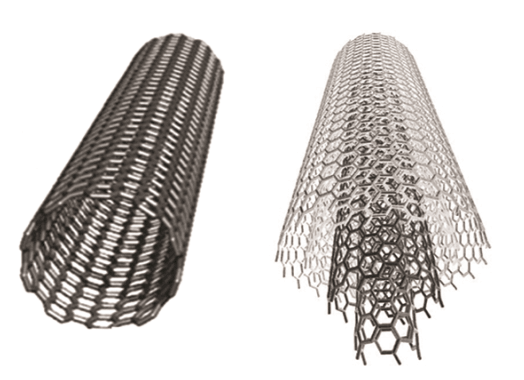
Figure 2: Schematic representation of a single-walled nanotube (SWNT) and a multiwalled nanotube (MWNT)2
Carbon nanotubes have found many uses in energy applications. Carbon nanotubes can store hydrogen, enable electrons to flow, or increase catalyst activity. In fuel cell research, carbon nanotubes have been added to the platinum/carbon catalyst mixture at the anode to improve the efficiency of the catalyst reactions in the fuel cell. Nitrogen-doped carbon nanotubes have also been used to reduce the oxygen at the cathode in fuel cells. Through vertical alignment, carbon nanotubes can reduce oxygen in the alkaline solution more effectively than platinum.
Research has been on-ongoing with carbon nanotubes used to store small amounts of hydrogen at ambient temperatures and pressures. Several groups have investigated this technology as hydrogen storage to reach the U.S. Department of Energy benchmarks of 62 kg H2/m3 and 6.5 percent weight of stored hydrogen to total system weight for hydrogen fuel cell vehicles. Graphitic nanofibers, single-walled carbon nanotubes (SWNTs), and multiwalled carbon nanotubes (MWNTs) have been investigated for hydrogen storage and have a wide range of hydrogen absorption values. Table 1 shows the estimated storage of a few carbon-based absorbents for an automobile.
| Vessel Type | Li-doped Carbon Nanofibers | K-doped Carbon Nanofibers | 30 wt% Carbon Nanofibers |
| Volume (L) | 33 | 51 | 24 |
| Weight (kg) | 43 | 66 | 35 |
| H2 | 5 | 5 | 5 |
| E/V (MJ/L) | 15 | 10 | 28 |
| E/m (MJ/kg) | 11 | 7.8 | 19 |
Table 1: Estimated Storage of Carbon-Based Absorbants Designed for a 640-km Range in a 34 km/L Gasoline Equivalent Car3
No consensus has yet been made between scientists on the amount of hydrogen that graphitic nanofibers, SWNTs, and MWNTs can hold. Experimental results are often difficult to interpret due to material purity and minute differences in experimental technique. More recent studies on carbon nanotubes are beginning to show some consistency and reproducibility in experiments. This topic is somewhat controversial, and the practical feasibility of hydrogen storage with carbon nanotubes still needs to be proven.
In solar cells, cells have been developed using a mixture of carbon nanotubes and carbon Buckyballs. Buckyballs can trap electrons, but they cannot make electrons flow. Since nanotubes act like copper wires, they can make the electrons flow to the load.
Carbon nanotubes are some of the strongest and stiffest materials ever discovered. This high-strength make them useful for improving the strength of other non-solid structures and materials. A study published in the journal, Nature, determined that carbon nanostructures are probably present in high-strength steel and this was probably one of the factors that accounted for the legendary strength of ancient swords.
Due to carbon nanotube’s high-strength and superior mechanical properties, nanotubes can be incorporated into materials to promote high strength and unmatchable toughness. One possibility that could bring all types of new engineering designs and technologies into development is putting carbon nanotube molecules into a polymer matrix to form a super high-strength composite material. This would enable extremely high-pressure hydrogen storage for fuel cells, super-bullet-proof vests and clothing, and many other types of new technologies.
Carbon nanotubes have useful applications as fuel cell catalysts, hydrogen storage as well as countless other applications. The main roadblock that prohibits the widespread use of carbon nanotubes in novel applications is the economics of the synthesis process. Therefore, future research should focus on the creation of a cost-effective process to mass produce carbon nanotubes. Due to its proven stability and electrical and mechanical properties, wide-spread adoption of carbon nanotubes into many applications would result, which in turn would yield numerous improvements in materials and energy applications.
1 Dyatlova, Olga & Gomis-Bresco, Jordi & Malic, Ermin & Telg, Hagen & Maultzsch, Janina & Zhong, Guofang & Geng, Junfeng & Woggon, Ulrike. (2012). Dielectric screening effects on transition energies in aligned carbon nanotubes. Physical Review B. 85. 10.1103/PhysRevB.85.245449.
2 "Carbon Nanotubes - Polymer Nanocomposites", book edited by Siva Yellampalli, ISBN 978-953-307-498-6, Published: August 17, 2011 under CC BY-NC-SA 3.0 license.
3 Klos, H. “Technical and Economic Practicability of Carbon Nanostructures Hydrogen Storage Systems.” 12th World Hydrogen Energy Conference. Vol. 2, 1998, pp. 893–898. Pettersson, Joakim and Ove Hjortsberg. “Hydrogen Storage Alternatives–A Technological and Economic Assessment.” December 1999. Volvo Teknisk Utveckling AB. Chen, P, X. Wu, J. Lin, and K.L. Tan. “High H2 Uptake by Alkali-Doped Carbon Nanotubes under Ambient Pressure and Moderate Temperatures.” Science. Vol. 285, No. 5424, 1999, pp. 91–93.

 Posted by
Posted by


















Enter the code in the box below: