 Fuel cell operating conditions depend upon the cell and stack design. The operating parameters that affect fuel cell performance are:
Fuel cell operating conditions depend upon the cell and stack design. The operating parameters that affect fuel cell performance are:
• Operating Pressure
• Operating Temperature
• Flow Rates of Reactants
• Humidity of Reactants
Using the correct operating condition for each parameter is critical to obtain good fuel cell performance. We will discuss these operating conditions in the remainder of this post.
A fuel cell can be operated at ambient pressure or in a pressurized state. Fuel cell performance often improves with increased pressure. However, the need for gas compression and storage may make the system less efficient. Pressurization of the fuel also changes the water management in each cell; therefore, the fuel cell operating conditions must be analyzed from a system perspective. Fuel cell parameters such as inlet fuel compositions, the diffusivity of the gas diffusion layer (GDL) and the flow-field plate designs may change with reactant gas pressure. Since the saturation pressure does not vary for constant operating temperature, the mole fraction of an oxidant such as oxygen may increase with increasing operating pressure.
A higher operating temperature creates greater fuel cell efficiency. For each fuel cell design, there is an optimal temperature, and the operating temperature must be specifically chosen for each fuel cell system. A fuel cell generates heat as a by-product of the electrochemical reaction, and this heat must be controlled to maintain the desired temperature. The designed operating temperature of the fuel cell affects many factors. A higher operating temperature means more of the product water is vaporized so the waste heat goes into the latent heat of vaporization and less liquid water is pushed out of the fuel cell. Higher temperatures also mean faster kinetics and a voltage gain that exceeds the voltage loss from the negative thermodynamic relationship between the open-circuit voltage and temperature. Lower temperatures equal shorter warm-up times for the fuel cell system and lower thermomechanical stresses. Corrosion and other time- and temperature-dependent processes are reduced so much less water is required for the saturation of input gasses. The upper limit of operation for PEMFCs is approximately 90 ºC because water evaporates from the membrane which dries the membrane out and causes the performance to drop quickly.
The flow rate of the reactants must be equal to, or greater than, the rate at which those reactants are consumed within the cell. Oxygen and hydrogen are introduced into the fuel cell system at the appropriate flow rate necessary for the required current. This requires a variable–flow system if the stoichiometry is to be kept constant. Even in a system that uses “atmospheric pressure”, a pressure slightly above atmospheric is needed to push the gasses through the flow fields and force the liquid water out. The additional pressure required is 0.1 to 2.0 psi (0.7 to 13.8 kPa) above atmospheric.
In PEM fuel cell stacks, the reactant gasses need to be humidified before entering the cell for high ionic conductivity of the membrane. The polarization curves for different operating levels of air and hydrogen relative humidity are shown in Figure 1. The best performance was obtained when hydrogen was at 70-percent humidity. At high current densities, the transport from the anode by electro-osmotic drag exceeds transport to the anode by back diffusion from the cathode, which leads to membrane dehydration and performance degradation. Air that has a low humidity can exacerbate this effect by reducing the rate of back diffusion from the cathode. Humidifying the anode gasses leads to higher levels of fuel cell performance. When air has high humidity levels, the performance only improves slightly due to the back diffusion of the water at the cathode.
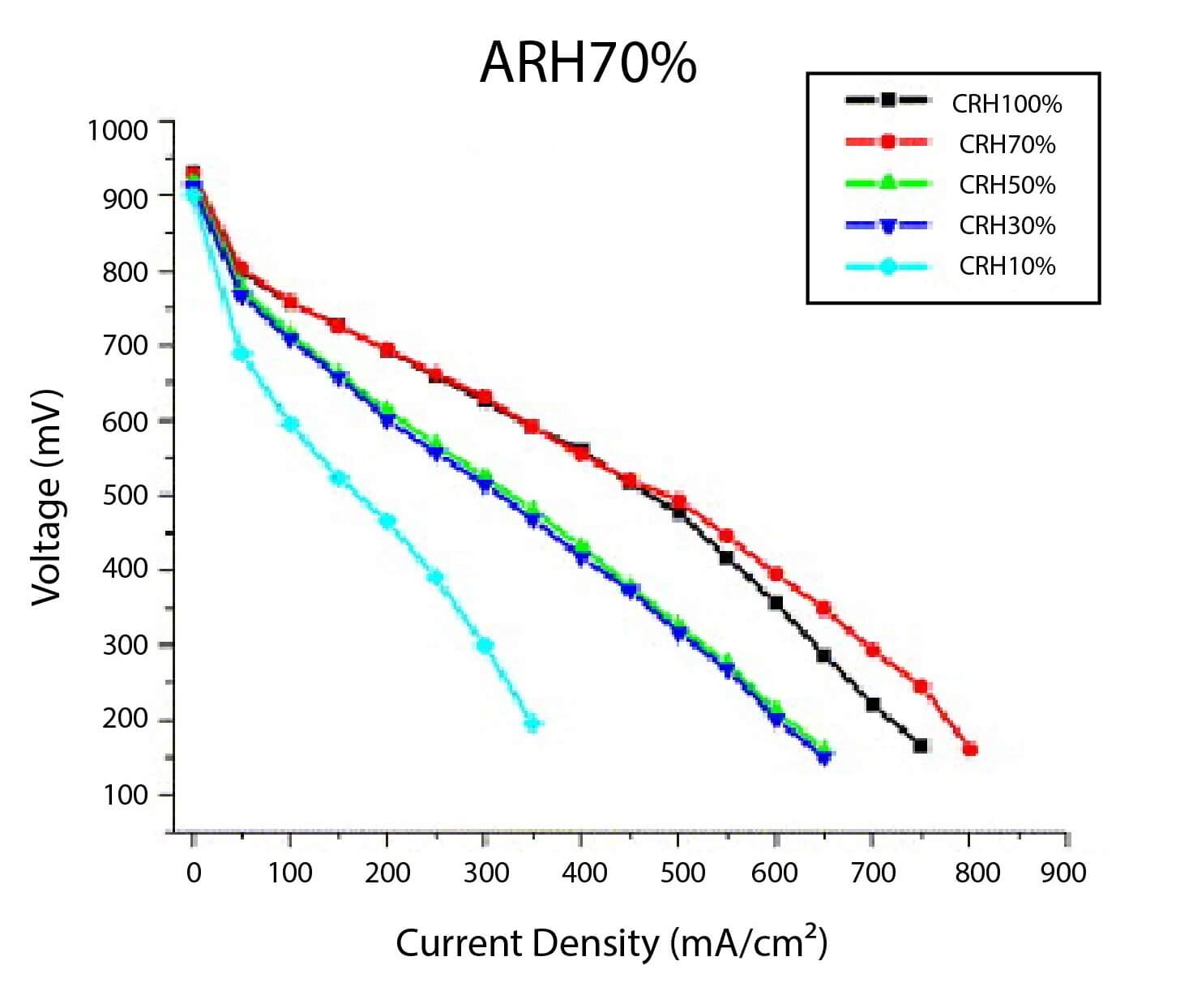
Figure 1. Polarization curves as function of feed gas humidity fuel cell [1].
• A flowchart must be drawn and labeled. Enough information should be included on the flowchart to have a summary of each stream in the process. This includes known temperatures, pressures, mole fractions, mass flow rates and phases.
• Write the appropriate mass balance equation to determine the flow rates of all stream components, and solve for any desired quantities.
Fuel cell operating conditions depend upon the fuel cell and stack design. The optimal conditions will vary from cell to cell; therefore, the optimal parameters need to be studied and preferably calculated to operate the cell. The typical operating conditions are different for each fuel cell type. PEMFCs typically operate between 20 and 100 ºC, and from 1 to 3 atm. The flow rates vary depending upon the fuel type and oxidant used. Higher humidity’s typically improve the performance of PEMFCs. DMFCs also operate from 20 to 100 ºC and from 1 to 3 atm. Flow rates vary depending upon the concentration of methanol in water and the system design. Using the correct operating conditions is necessary for obtaining good fuel cell performance.
L.A.M. Riascos, M.G. Simoes, Paulo, Eigi Miyagi, Mello Moraes. Controlling PEM Fuel Cells Applying a Constant Humidity Technique. Electrochimica Acta, Jan 2008.

 Posted by
Posted by



































Enter the code in the box below: