Article Search
SearchFuel Cell Store Blog
Ion exchange materials are used to purify, separate, and extract many different types of molecules, including organic and biochemical molecules. When ion exchange materials involve these ion types, there may be additional complexities involved with the interaction.Some of the phenomena that may occur are:
- Secondary forces between the ionized group and counterion. These forces may consist of coordination, hydrogen, and van der Waals bonding.
- The pH can affect the percent ionization.
- The position of the functional groups can affect ion transport.
- Hydration of organic molecules can be more complex than inorganic ions.
- Organic ions may be larger than inorganic ions; thus, steric hinderances can reduce ionic interactions.
Therefore, ion exchange phenomena may be able to be explained chemically by stoichiometric reactions, but the actual ionic selectively may be determined by other interactions.

Thanks to our handy Hydrogen Air Calculator Sheet, you can take the IV curve of any membrane electrode assembly (MEA), assign an active area, current density, and desired power output and the calculator will determine the number of MEAs needed along with the voltage and current of the fuel cell operating at that point.

Membranes are essential for PEM fuel cells to operate. The Proton Exchange Membrane carries the hydrogen ions from the anode to the cathode without passing the electrons that were removed from the hydrogen atoms.
The Fuel Cell Store carries the largest selection of Membranes in the world! We help you compare all the Membranes we offer in one simple file so you can narrow down the perfect Membrane for your project. With our Membrane Comparison Table you can compare the specifications of all our Cation Exchange Membranes, Anion Exchange Membranes, and Bipolar Membranes with ease.

Gas Diffusion Layers (GDL) are one of the components in different types of fuel cells including, but not limited, to Proton Exchange Membrane and Direct Methanol fuel cells. Gas Diffusion Layers serve to provide conductivity in the cell and control the contact between the reactant gases and the catalyst. This layer also aids in managing the water transport out of the membrane. Another essential function of a GDL is to provide a connection between the membrane electrode assembly and graphite plates in the fuel cell stack.



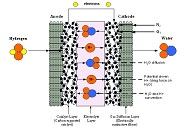
A numerical model was developed to predict the water concentration, temperature, potential and pressure across a Nafion membrane used in proton exchange membrane (PEM) based fuel cells. The numerical model consists of simultaneously calculating the diffusive flux for water and hydrogen, the proton potential and the pressure and temperature at each node...
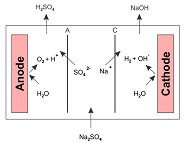
Ion-exchanges membranes (IEMs) have many applications beyond fuel cells -- they can also be used to synthesize all types of compounds that are used in various industries. The most popular IEMs consist of polymeric resins with charged functional groups based upon their ion selectivity, they are referred to as anion-exchange (AEM) and...

Anion exchange membranes (AEMS) have been an active area of research for over a decade. AEMS can be used for fuel cells, redox flow batteries, electrolyzers, and even water desalination membranes. The electrolyte layer is the “heart” of electrochemical cells such as fuel cells, batteries, and because it transports ions from...
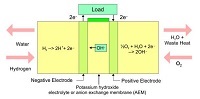
Alkaline fuel cells (AFCs) was one of the first extensively researched fuel cell types and was used by NASA for the Gemini, Apollo, and Space Shuttle missions. The first alkali electrolyte fuel cell was built by Francis Thomas Bacon (1904–1992) in 1939. He used potassium hydroxide for the electrolyte and...

Direct methanol fuel cells (DMFCs) utilize a mixture of methanol and deionized water (or distilled water) as the fuel for anode side. The most common range for the molarity of the methanol is 0 to 1 Molar and occasionally 0 to 2 Molars (the latter one for advanced users utilizing customized MEAs or CCMs). Our MEAs or CCMs that are manufactured for DMFCs...
Many countries around the world have been diligently working towards implementing renewable energy plants for over a decade. According to the International Energy Agency (IEA), renewables in the form of hydropower, bioenergy, wind and solar will account for 18% of primary energy by 2035. Since 2013, more electrical grid capacity was added...

This is the fifth and final part in a SaveOnEnergy series discussing the ins and outs of different forms of renewable energy. In this series, we’ve covered a range of renewable power sources. Some, such as geothermal power, have been commonly used for decades. Others, including solar power, have recently risen in popularity due to...








