Quattro Electrolyzer
The Quattro Electrolyzer is a proton exchange membrane (PEM) based electrolyzer hardware that is comprised of four electrolyzer cells for the production of hydrogen and oxygen. The electrolyzer technology is one of the most promising clean technologies for creating hydrogen and oxygen gases from the water electrolysis reaction. It is an excellent educational tool for teaching students at all levels (whether K-12, pre-college, college or vocational schools, or university) or for hobbyists to learn for how to scale up the electrolysis concept for increased output. Stacking up more of the electrolyzer cells in the design makes this electrochemical hardware will vividly show to students or hobbyists how larger hydrogen and oxygen gas generation could be achieved from an engineering perspective. Please see the technical specs for hydrogen and/or oxygen gas production levels.
This electrolyzer product is maintenance-free. However, always remember to use fresh, distilled water each time and to drain the water from the storage tanks after use.
• Standard Hydrogen Production Rate: 46 cm3/min (with room temperature de-ionized or distilled water)
• Maximum Hydrogen Production Rate: 50 cm3/min (with warmed water around 42-45 deg. Celsius)
• Standard Oxygen Production Rate: 23 cm3/min (with room temperature de-ionized or distilled water)
• Maximum Oxygen Production Rate: 25 cm3/min (with warmed water around 42-45 deg. Celsius)
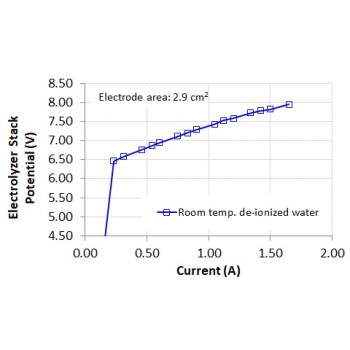
• Standard operating current: 1.65 A (with room temperature de-ionized or distilled water)
• Stack Voltage at the standard operating current: 7.9 V to 8.0 V (measured at the stack terminals, with room temperature de-ionized or distilled water)
• Maximum Operating Current: 1.80 A (with warmed water around 42-45 deg. Celsius)
• Stack Voltage at the maximum operating current: 7.9 V to 8.1 V (measured at the stack terminals, with warmed de-ionized or distilled water at 42-45 deg. Celsius)
• Weight: 2.9 oz
• Dimensions (H x W x D): 3.0" x 2.1" x 2.2" (75 x 71 x 53 mm)
• Requires the use of commercial de-ionized water (or commercial distilled water) with a conductivity of < 2 μS/cm
The growing significance of PEM electrolyzers mirrors the development of fuel cells. Electrolyzers generate the hydrogen required by fuel cells from de-ionized water (or distilled water) in an environmentally conscious manner. The electrical energy required for this purpose can be gained from renewable sources such as solar cells, wind farms, or hydroelectric plants.
Water reacts in the electrolyzer under the influence of electrical energy according to the following formula: 2H2O = 2H2 + O2. This process takes place in the MEA (membrane electrode assembly). The MEA consists of a cathode, an anode, and a special polymer membrane (PEM) coated with catalysts which is permeable to protons but which presents a barrier to electrons. Your H-tec quattro electrolyzer functions on the PEM (proton exchange membrane) principle. The gases produced can be collected in storage tanks (such as H-TEC Storage 30 or H-TEC Storage 80). The energy stored in chemical form in the gases can be converted back to electrical energy in a fuel cell as and when required.
Write a review
Your Name:
Your Review: Note: HTML is not translated!
Rating: Bad Good
Enter the code in the box below:









 H-TEC Education E105 Quattro Electrolyzer Manual
H-TEC Education E105 Quattro Electrolyzer Manual