Catalyst
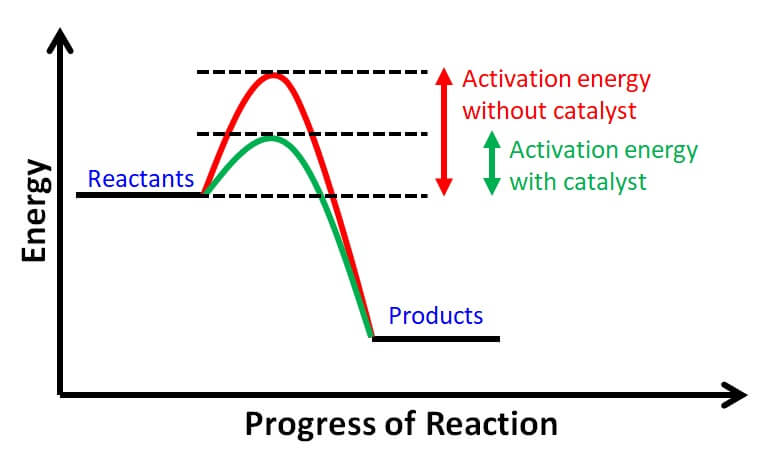
Electrochemical devices have to utilize catalyst materials in order to function and deliver high performance. There exist numerous catalyst materials for various reactions. In any electrochemical devices, an oxidation reaction needs to couple with a reduction reaction. Examples of such reactions are hydrogen oxidation reaction (HOR), hydrogen evolution reaction (HER), oxygen reduction reaction (ORR), water oxidation (which is also called water electrolysis), etc. Catalyst materials reduce the activation energy of reactions and hence enable manufacturing of highly efficient electrochemical devices that achieves wonders.
Platinum, platinum alloys with transition metals, platinum-ruthenium alloy, palladium and other similar catalytic materials have been the most commonly utilized catalyst materials for various electrochemical devices such as fuel cell, electrolyzers, electrochemical pumps (also known as electrochemical compressors), electrochemical inerters, electrochemical dehumidification, etc. Catalyst materials can be manufactured either in black (also known as pure form) or in the supported form. Black (or pure form) of the catalytic materials would usually have lower surface area compared to their supported counterparts. Carbon has been the most promising support material due to its extremely large surface area, good electrical conductivity, and inherent inertness in fuel cell devices.
Fuel cells create electrical energy (or power) simply by oxidizing the fuel species (such as hydrogen molecules) to protons and electrons on the anode electrode and reduce the oxygen molecules to water by electrochemically combining surface adsorbed oxygen species with protons/electrons on the cathode electrode.
FuelCellStore has more than 100+ catalytic materials in its inventory (a partial list of them provided here) and has the know-how for fabricating various membrane electrode assemblies (MEAs) or catalyst coated membranes (CCMs). FuelCellStore supplies the following catalytic materials due to their most common use in electrochemical devices: platinum based catalysts, platinum ruthenium based catalysts, palladium based catalysts, iridium based catalysts and other catalysts (Ag, Au, Co, Cu, Fe, Ni, Rh, Ru, and Sn).
Generally, in a polymer electrolyte membrane fuel cell (PEMFC or proton exchange membrane fuel cell), platinum black or platinum supported on carbon are used as the catalyst both for anode and cathode sides. In PEM electrolyzers, iridium ruthenium oxide, iridium oxide, iridium black, platinum black can be used as the anode catalyst. For the cathode side of the electrolyzers, platinum black or platinum supported on carbon catalysts can be used. Direct methanol fuel cell (DMFC) requires the addition of ruthenium as a catalyst to the platinum (also called as platinum-ruthenium alloy) in order to facilitate the electrochemical oxidation of alcohol fuel.



















