Bipolar Membranes (BPM)
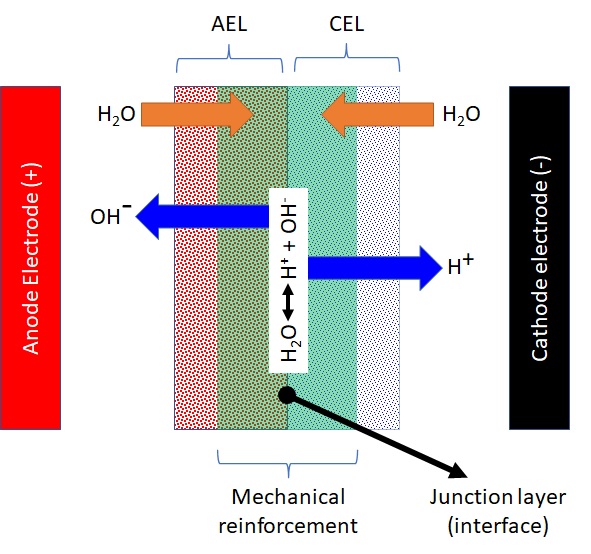 Bipolar membranes are in general comprised of a mechanical reinforcement that is sandwiched between a cation exchange layer (CEL) and an anion exchange layer (AEL). Cation exchange layer (CEL) is formed by the cation exchange dispersion on one side of the mechanical reinforcement. An anion exchange layer (AEL), on the other hand, is formed from the use of an anion exchange dispersion on the opposite side of the mechanical reinforcement. A composite bipolar membrane can also be called as composite bilayer membrane.
Bipolar membranes are in general comprised of a mechanical reinforcement that is sandwiched between a cation exchange layer (CEL) and an anion exchange layer (AEL). Cation exchange layer (CEL) is formed by the cation exchange dispersion on one side of the mechanical reinforcement. An anion exchange layer (AEL), on the other hand, is formed from the use of an anion exchange dispersion on the opposite side of the mechanical reinforcement. A composite bipolar membrane can also be called as composite bilayer membrane.
In the intermediate layer between Anion Exchange Layer (AEL) and Cation Exchange Layer (CEL), water is dissociated into OH- and H+ ions when exceeding a potential difference of approximately 0.8 V. The CEL must be directed towards the cathode, the AEL must be directed towards the anode, and the mode of operation has to be reverse biased in order to promote the water dissociation reaction. Under the reverse biased mode, the electrons would be transferred from anode side to cathode side. Water molecules would naturally diffuse into the intermediate layer between AEL and CEL and generation of H+ and OH- ions would occur as a result of water splitting reaction. H+ ions will diffuse out from the CEL layer and migrate into the cathode chamber. OH- ions, on the other hand, would diffuse out from the AEL layer and migrate into the anode chamber.
The following applications utilizes bipolar membranes: Water splitting, electrodialysis, production of acids and alkali from a corresponding salt which is also known as salt splitting reaction, and other similar electrochemical technologies or devices.
Figure (a) provides the schematic representation of the composite bipolar membrane under reverse bias mode, where first the junction is depleted of ions and then water dissociates into H+ and OH- ions. Figure (b) describes the operation of a composite bipolar membrane under forward bias mode, where H+ and OH- ions are transported into the bipoplar membrane through their respective layers and water is formed at the bipolar junction (also called as bipolar interface or interface layer). AEL stands for anion exchanger layer, CEL stands for cation exchange layer, IL stands for interface layer.
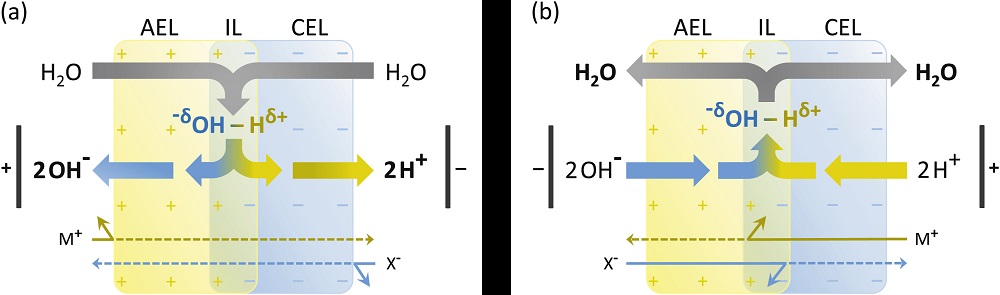
[This descriptive figure/image is from Pärnamäe et. al (January 2021), article entitled as "Bipolar membranes: A review on principles, latest developments, and applications", and can be found here: https://doi.org/10.1016/j.memsci.2020.118538 ]
The article by Pärnamäe et al. entitled "Bipolar membranes: A review on principles, latest developments, and applications" is considered to be an excellent source that describes the operating principle of bipolar membranes, provides a very through analysis of the recent progress in the area of bipolar membranes and use of such membranes in various applications.
The article by Jaroszek and Dydo entitled "Ion-exchange membranes in chemical synthesis - a review" is considered to be an excellent source for how to properly use a composite bipolar and other ion exchange membranes for various chemical synthesis reactions via electrodialysis, 2-chamber membrane electrolysis, 3-chamber electro-electrodialysis, 4-chamber electrodialysis metathesis, electrodialysis with bipolar membrane, electrodeionization, ion substitution electrodialysis, Donnan dialysis, etc.










