 Fuel cells usually use compressed hydrogen as the fuel; however, there are many liquid fuels that can be used with fuel cells. Depending upon the system design, liquid fuel types may not be as efficient as pure hydrogen because the hydrogen needs to be “stripped” off the fuel molecule and then used in the fuel cell. The hydrogen can be stripped from the fuel molecule outside or inside the fuel cell. Some of the fuels that are processed inside the fuel cell include methanol, ethanol, and ammonia. These are fed directly into the fuel cell, and the hydrogen is stripped from the molecule via the platinum catalyst layer. These fuels are perceived to be safer by the public than hydrogen and are easy to transport and replenish when they are empty. One of the disadvantages of using liquid fuels is that they poison the catalyst layer over time. Methanol, ethanol, and ammonia can also be processed through a reformer outside of the fuel cell to avoid poisoning the fuel cell catalyst. It is easier in some fuel cell systems to replace the reformer (if necessary) instead of the catalyst layers inside the fuel cell. The remainder of this post describes the use of methanol, ethanol, and ammonia in greater detail.
Fuel cells usually use compressed hydrogen as the fuel; however, there are many liquid fuels that can be used with fuel cells. Depending upon the system design, liquid fuel types may not be as efficient as pure hydrogen because the hydrogen needs to be “stripped” off the fuel molecule and then used in the fuel cell. The hydrogen can be stripped from the fuel molecule outside or inside the fuel cell. Some of the fuels that are processed inside the fuel cell include methanol, ethanol, and ammonia. These are fed directly into the fuel cell, and the hydrogen is stripped from the molecule via the platinum catalyst layer. These fuels are perceived to be safer by the public than hydrogen and are easy to transport and replenish when they are empty. One of the disadvantages of using liquid fuels is that they poison the catalyst layer over time. Methanol, ethanol, and ammonia can also be processed through a reformer outside of the fuel cell to avoid poisoning the fuel cell catalyst. It is easier in some fuel cell systems to replace the reformer (if necessary) instead of the catalyst layers inside the fuel cell. The remainder of this post describes the use of methanol, ethanol, and ammonia in greater detail.
Methanol (CH3OH) is an alcohol-based fuel with an energy density much greater than compressed hydrogen. Methanol is an attractive fuel for fuel cells because the supply chain can easily provide methanol for fuel cells. Approximately 90 percent of the world’s methanol is manufactured from synthesis gas from natural gas. Methanol can also be produced from non-petroleum feed-stocks such as coal and biomass. Approximately 75 percent of methanol is used for chemicals, and the remaining 25 percent is used for fuels.
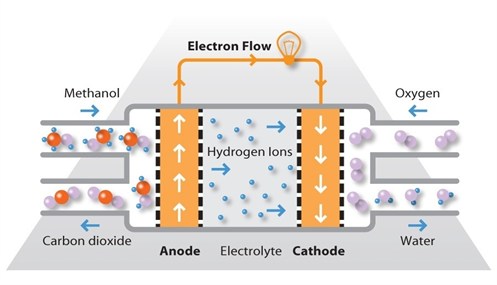
• Methanol has a higher energy density compared to hydrogen.
• It is perceived to be a safer fuel than hydrogen.
• It may lower hydrogen infrastructure costs.
Some studies indicate that methanol-powered fuel cells (DMFCs) are the best option for commercializing fuel cells because the current infrastructure does not have to change as much as for hydrogen. There is also no adverse perception of methanol and a large amount is already manufactured. Other studies indicate that there will need to be as much spent on setting up a methanol infrastructure as the hydrogen infrastructure.
Ethanol is an attractive fuel for fuel cells because the supply chain is already in place, and is easier to work with for consumers. Ethanol is a hydrogen-rich liquid that has a high-energy density compared to methanol (8.0 kWh/kg versus 6.1 kWh/kg). Ethanol can be produced from a variety of feedstocks. In the United States, it is produced mainly from corn by a cooking, fermentation, and distillation process. Brazil uses sugarcane as the primary feedstock. Plants that have higher energy yields, such as switchgrass and sugarcane are more effective in producing ethanol than corn. Future candidates for ethanol production include cellulose feedstocks and agricultural wastes.
• Ethanol has a high-energy density compared to hydrogen.
• It is perceived to be a safer fuel than hydrogen.
• It may lower hydrogen infrastructure costs.
Since ethanol can be derived from a variety of biological sources (as well as through fossil fuels), this fuel is a good option for overcoming the storage and infrastructure challenges for fuel cells.
Ammonia reforming is a thought-provoking option because it offers clean combustion from a chemical feedstock that is commercially available as a fertilizer. Ammonia contains 17.6 percent hydrogen atoms by weight, which is similar to the weight content of methanol when reformed using the partial oxidation reformation. Ammonia reforming also has the advantage of only having hydrogen and nitrogen gas as the by-products. Ammonia is easily liquefied under pressure with a liquid density of 601 g/L at 300 K (equivalent to an hydrogen volumetric density of 55 g/L). The liquefaction requires a pressure of only 10 bar at 300 K.
The ammonia cracking reaction is: NH3 ⇒ 1/2 N2 + 3/2 H2 (⌂H = +46.4 kJ / mol NH3)
The reaction takes place at over 400 ºC, which requires an external heat source since the exhaust from a traditional PEM exits at only 80 ºC. Some of the hydrogen in the reformer’s output stream can be burned to provide the necessary temperature for the reformer and to provide the heat needed for cracking. It is also possible to tune the anode utilization of the fuel cell, so the exhaust stream from the fuel cell has enough heating value from the unconsumed hydrogen to supply the required heat if burned.
An issue with using ammonia as a fuel is the undissociated ammonia concentration in the product gas. Although the concentration is less than 50 ppm, this is still enough to damage fuel cells with acid electrolytes, so an acid scrubber is needed to remove the final traces of ammonia gas from the cracker.
Many types of fuels can be used for different fuel cell types and systems. The cleanest fuel type is hydrogen, but there are many fuel cell designs that can utilize other fuels. Common fuels include hydrogen, methanol, ethanol, and ammonia. The use of fuels other than hydrogen may be beneficial for the commercialization of fuel cells in the near term, but the overall goal of fuel cell technology is to use pure hydrogen from renewable sources of energy other than fossil fuels.

 Posted by
Posted by























